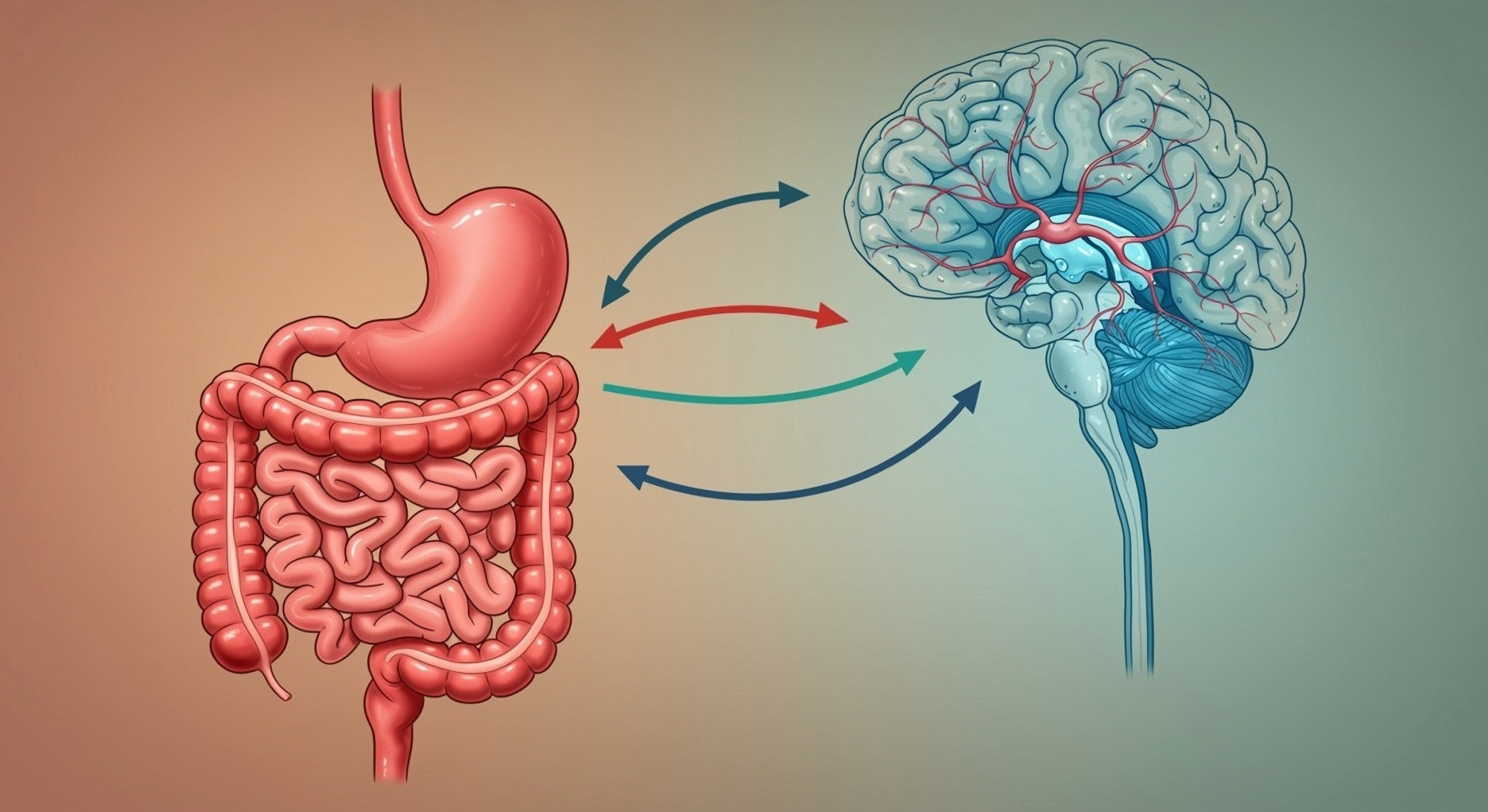
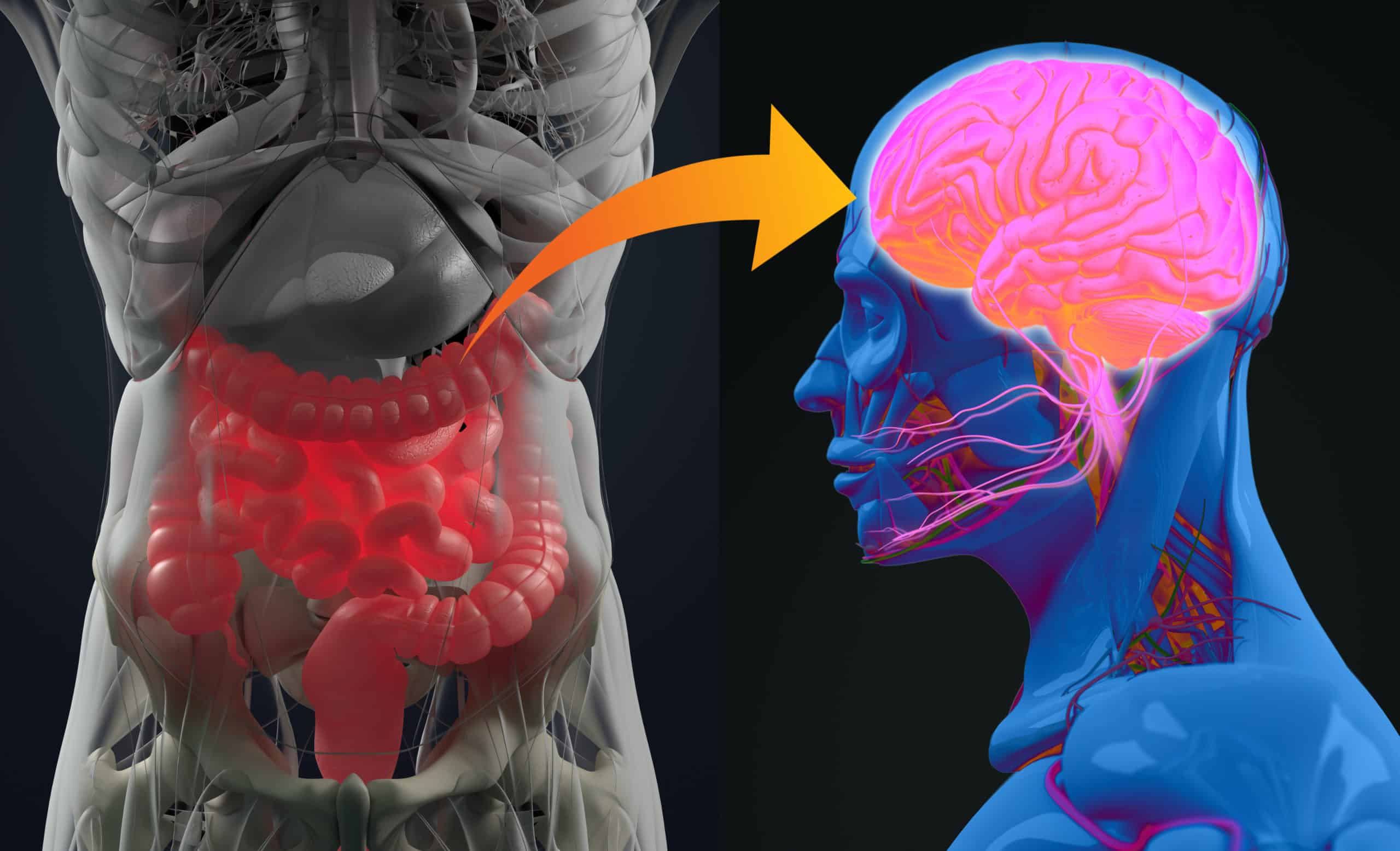
Recent research has uncovered a compelling link between gut health and autism spectrum disorder (ASD), suggesting that the gut-brain axis may play a pivotal role in influencing behavior, cognition, and emotional regulation in individuals with autism. A study from USC highlights altered gut metabolites, such as kynurenate, and their connection to brain activity patterns and ASD severity. These findings open new avenues for treatment, including dietary interventions, probiotics, and even fecal microbiota transplantation (FMT), which could complement traditional therapies. As gastrointestinal issues are common in ASD, integrating gut health assessments into autism care could significantly enhance outcomes. With more research, personalized therapies targeting the gut-brain connection may offer a holistic, transformative approach to managing autism.
Gut-Brain Connections: A New Frontier in Autism Care
Intended Audience and Purpose of the Article
Audience:
- Parents and caregivers of individuals with autism spectrum disorder (ASD)
- Healthcare professionals and therapists working in autism care
- Researchers and students interested in neuroscience, microbiome, or behavioral health
- Public health advocates and policymakers
- Donors and changemakers interested in supporting innovative, inclusive healthcare research
Purpose:
To translate emerging scientific evidence linking gut health and brain function into accessible insights and to explore its potential implications for autism care. This article aims to raise awareness, spark critical discussion, inspire future research, and encourage holistic approaches to managing ASD, grounded in both compassion and science. Our goal is to empower those affected by autism—whether personally or professionally—with knowledge that bridges cutting-edge research and real-world application.
I. Executive Summary: A New Frontier in Autism Care
The Gut May Hold the Key to Unlocking New Avenues in Autism Support
A groundbreaking shift is emerging in the understanding and treatment of autism spectrum disorder (ASD)—and it begins not in the brain, but in the gut. While traditional models of autism care have primarily focused on behavioral therapy, educational support, and medication, a growing body of research now points to the gut-brain axis as a potential game-changer in understanding the roots of autism-related challenges and opportunities for care.
Recent studies, including a comprehensive and interdisciplinary research initiative by the University of Southern California (USC), are shedding light on how imbalances in the gut microbiome and gut-derived metabolites are closely linked to altered brain activity and behavioral symptoms in children with autism. These findings suggest that what happens in the digestive tract may have profound implications for emotional regulation, sensory processing, social behavior, and cognitive development.
This emerging science challenges us to rethink autism as more than a neurodevelopmental disorder confined to the brain. Instead, we are invited to embrace a systems-level perspective—where the microbiome, metabolism, neurology, and behavior are all interconnected in a complex, dynamic web.
The Gut-Brain Connection: From Insight to Intervention
At the heart of this new understanding is the gut-brain axis—a bidirectional communication system where the gut microbiota influences brain chemistry and vice versa. Researchers have identified that critical neurotransmitters like serotonin, which affects mood, anxiety, learning, and social behavior, are not only active in the brain but are also primarily produced in the gut.
The USC study specifically examined altered gut metabolites, such as those in the tryptophan pathway, which includes serotonin and kynurenate. These chemicals are known to play vital roles in neuroplasticity, immune function, and emotional processing. The study found that lower levels of these metabolites in children with ASD were associated with differences in brain activity and severity of autism symptoms.
Even more compelling is the identification of specific brain regions—notably the mid-insula and mid-cingulate cortex—where activity patterns appear to be shaped by gut chemistry. These areas are crucial for processing emotions, empathy, and social cues, all of which are frequently impacted in individuals with autism.
Toward a Holistic, Hopeful Future
What does this mean for families, clinicians, and caregivers? It means that autism care may benefit from expanding beyond behavioral interventions to include gut-focused strategies, such as dietary changes, prebiotic and probiotic support, microbiome therapies, and potentially even metabolite-targeted treatments.
This new frontier is not without challenges—more research is needed, and many questions remain unanswered—but the potential for more effective, personalized, and holistic care is enormous. It offers a message of hope grounded in biology: that by nurturing the gut, we may help nurture the mind, too.
We are standing at the cusp of a paradigm shift—one that could transform how we understand, treat, and support individuals with autism. And with that shift comes the responsibility to stay informed, advocate for integrative research, and work together to co-create a future where science and compassion walk hand in hand.
II. Understanding Autism and the Gut-Brain Axis
Autism Spectrum Disorder: A Complex Neurodevelopmental Condition
Autism spectrum disorder (ASD) is a neurodevelopmental condition that affects how a person perceives the world, processes information, and interacts with others. It is typically diagnosed in early childhood and manifests across a broad range of abilities and challenges—hence the term “spectrum.”
While each individual with autism is unique, core behavioral characteristics often include:
- Social communication challenges: Difficulty in understanding social cues, forming relationships, or engaging in back-and-forth conversations.
- Language and communication difficulties: Ranging from complete absence of speech to highly advanced but rigid or idiosyncratic language use.
- Repetitive behaviors and restricted interests: Insistence on sameness, repetitive motor movements, intense focus on specific topics or routines.
- Sensory sensitivities: Over- or under-reactivity to sounds, lights, textures, or other sensory inputs.
These behaviors are often accompanied by co-occurring conditions, such as anxiety, ADHD, gastrointestinal issues, and sleep disturbances—suggesting that autism is not just a brain-based disorder but involves multiple physiological systems.
What Is the Gut-Brain Axis?
The gut-brain axis refers to a sophisticated, bidirectional communication system between the central nervous system (CNS) and the enteric nervous system (ENS)—often called the “second brain”—which governs the gastrointestinal tract.
This axis operates via:
- Neural pathways (especially the vagus nerve),
- Hormonal signaling (including gut hormones),
- Immune system interactions, and
- Microbial metabolites produced by trillions of gut-residing microorganisms (the gut microbiota).
In simple terms, the gut and the brain are in constant conversation. Signals from the gut can influence mood, cognition, stress response, and even social behavior. Remarkably, over 90% of the body’s serotonin—a key neurotransmitter involved in mood regulation—is produced in the gut, not the brain.
Gut-Brain Axis and Autism: The Missing Link?
In recent years, scientists have started to uncover how dysregulation in the gut-brain axis may contribute to autism-related symptoms—particularly in domains like:
- Emotional regulation (difficulty with mood swings, frustration tolerance),
- Social behavior (empathy, bonding, recognition of emotions),
- Sensory processing (heightened or dulled responses to stimuli),
- Cognitive function (attention, learning, adaptability).
These are precisely the areas where many individuals with ASD experience challenges.
Studies have shown that children with autism often have a distinct composition of gut microbiota compared to neurotypical peers. This microbial imbalance (or dysbiosis) may result in the impaired production of key neuroactive substances, altered immune responses, and chronic low-grade inflammation—all of which can influence brain development and behavior.
Moreover, the immune and metabolic functions of the gut microbiome are now recognized as powerful mediators of neural plasticity, stress response, and even the expression of genes involved in brain development.
The Gut: A Neurochemical Powerhouse
To fully appreciate the gut’s role in neurological and psychological health, we must recognize that it is far more than a digestive organ. The gut:
- Produces neurotransmitters like serotonin, dopamine, and GABA.
- Regulates immune system responses, which are often heightened or altered in individuals with ASD.
- Controls systemic inflammation, which, when chronic, can affect brain functioning.
- Processes dietary nutrients into metabolites that directly or indirectly influence brain activity.
The tryptophan pathway, a focus of recent autism studies, is particularly notable. Tryptophan is a dietary amino acid that the gut metabolizes into compounds like serotonin and kynurenate. Altered tryptophan metabolism in the gut has been linked to anxiety, depression, and impaired cognition—and now, increasingly, to autism-related symptoms.
III. The USC Study: Revealing a Gut-Brain-Behavior Link in Autism
Bridging Biology and Behavior Through Interdisciplinary Research
A landmark study conducted by researchers at the University of Southern California (USC) marks a pivotal advancement in autism research. This groundbreaking work brought together multiple disciplines—metabolomics, neuroimaging, and behavioral science—to explore the deep-rooted physiological mechanisms linking the gut and brain in children with autism spectrum disorder (ASD).
The research, published in Nature Communications, sought to move beyond symptom observation and into the biochemical and neurological underpinnings of ASD. By studying both gut-derived metabolites and functional magnetic resonance imaging (fMRI) data alongside behavioral assessments, the team aimed to uncover whether—and how—gut imbalances are linked to altered brain function and observable autism-related behaviors.
This was not merely a correlation study, but an integrative analysis examining the mechanistic pathways by which the gut might influence the brain, and how that interaction manifests as behavioral traits characteristic of autism.
Key Findings: Connecting the Gut, Brain, and Behavior in Children with ASD
1. Reduced Levels of Key Tryptophan Pathway Metabolites in ASD
One of the most significant discoveries was that children with autism had markedly lower levels of certain metabolites in the tryptophan pathway, most notably kynurenate (KA). This pathway is crucial because it governs the metabolic fate of tryptophan—an amino acid that serves as a building block for neurotransmitters like serotonin and melatonin.
- Why this matters: KA and other tryptophan metabolites play critical roles in modulating excitatory neurotransmission, neuroinflammation, and synaptic plasticity—all of which influence behavior, mood, and cognition.
- Clinical insight: Lower KA levels suggest an impaired or diverted tryptophan metabolism in children with ASD, possibly contributing to neurochemical imbalances that affect emotional processing and social behavior.
2. Gut Metabolite Levels Corresponded with Altered Brain Activity Patterns
Using fMRI scans, researchers identified distinct differences in brain activity between children with ASD and neurotypical controls. Specifically, they observed abnormal activation in the mid-insula and mid-cingulate cortex—two brain regions deeply involved in:
- Emotional regulation
- Sensory integration
- Social cognition
- Interoception (the brain’s ability to interpret bodily sensations)
More strikingly, these changes in brain activity correlated with the levels of specific gut metabolites, particularly those derived from tryptophan.
- Interpretation: The findings suggest that gut chemical imbalances may directly influence brain function, which in turn shapes behavioral responses. For instance, low levels of indolelactate and tryptophan betaine were linked to heightened activity in regions governing disgust sensitivity and social aversion, symptoms frequently seen in ASD.
3. Behavioral Traits Were Mediated by Gut-Brain Signaling
Perhaps the most novel and compelling aspect of the study is the demonstration that the brain acts as a mediator—a kind of biochemical relay—between gut health and autism-related behaviors.
This means:
- Changes in gut metabolites didn’t just correspond to behaviors independently.
- Instead, they influenced specific neural circuits, and those circuits were directly tied to observable traits, such as:
- Disgust sensitivity (a strong reaction to certain textures, smells, or visuals),
- ASD severity scores, and
- Social withdrawal behaviors.
- Implication: This gut → brain → behavior model provides a new explanatory framework for autism that integrates physiological, neurological, and behavioral sciences in a way previously unexplored.
The Paradigm Shift: Autism as a Systems Condition
This study represents more than a set of biological observations—it signals a paradigm shift in our understanding of ASD:
- From viewing autism solely as a neurological disorder, to seeing it as a systems-level condition where the microbiome, immune system, metabolism, and brain are in continuous interaction.
- From treating behavioral symptoms in isolation, to exploring upstream causes and potential intervention points rooted in gut health.
In short, the brain is not just the source of autism symptoms—it is also a conduit through which the gut communicates its chemical signals, ultimately shaping how individuals with autism feel, perceive, and interact with the world around them.
IV. Implications for Future Therapies: Treating the Gut to Heal the Mind
A growing body of evidence suggests that targeting gut health may serve as a powerful adjunct—not a replacement—to current autism interventions, potentially offering more integrated, whole-person care for individuals with ASD. As we move toward a deeper understanding of the gut-brain-behavior connection, it opens exciting therapeutic frontiers that combine neuroscience, nutrition, microbiology, and personalized medicine.
This section explores four promising therapeutic avenues inspired by recent research, including the USC study. While some are still in their infancy or require further validation, all share a central theme: restoring gut balance to regulate brain function and behavior.
A. Dietary Interventions: Food as Medicine for the Gut-Brain Axis
What’s the Potential?
Certain dietary patterns—especially those that support gut microbial diversity, reduce inflammation, and nourish the intestinal lining—have been associated with improved mental and behavioral health outcomes. In the context of ASD, these include:
- Fiber-rich diets (vegetables, legumes, whole grains): Feed beneficial gut bacteria.
- Omega-3 fatty acids (fatty fish, walnuts, flaxseeds): Anti-inflammatory and critical for brain function.
- Polyphenols (berries, olive oil, cocoa): Antioxidant-rich and gut-friendly.
- Avoidance of ultra-processed foods: Minimizes gut barrier disruption and metabolic stress.
Caveat from the USC Study
The USC study found no significant differences in broad dietary categories between children with autism and neurotypical peers. However, this does not rule out the role of individual or targeted dietary strategies. The lack of effect at a population level might simply reflect the need for personalized nutritional profiling.
Actionable Insight:
- Work with nutritionists to tailor food plans to individual needs.
- Consider elimination diets (e.g., gluten-free, casein-free) cautiously and only with supervision.
- Focus on building long-term, sustainable eating habits rather than restrictive fads.
B. Prebiotics and Probiotics: Cultivating a Healthier Microbiome
What’s the Potential?
Prebiotics (non-digestible fibers that feed good bacteria) and probiotics (live beneficial microbes) have gained traction for their role in balancing the gut microbiome, improving digestion, and even modulating mood and anxiety levels.
- Lactobacillus and Bifidobacterium strains have shown some promise in influencing neurochemical levels, including GABA and serotonin.
- These interventions may also help with common GI issues in ASD, such as constipation or bloating.
Study Caveat:
The USC study excluded participants currently using prebiotic or probiotic supplements, so direct conclusions about their impact could not be drawn from this research. Nevertheless, other literature supports the therapeutic potential of microbiome-modulating supplements, especially when paired with dietary changes.
Actionable Insight:
- Consider microbiome testing before starting a supplement regimen.
- Explore synbiotics (combinations of prebiotics and probiotics) for synergistic effects.
- Monitor responses carefully—not all probiotics are beneficial for everyone.
C. Fecal Microbiota Transplantation (FMT): Resetting the Microbiome
What’s the Potential?
FMT involves transferring stool from a healthy donor into the GI tract of a recipient to reseed the gut with diverse, beneficial microbes. Though still considered experimental in many parts of the world, FMT has shown significant promise in early studies involving autism.
- A landmark study led by Dr. James Adams and colleagues (2017, 2019) showed that FMT improved both GI symptoms and behavioral symptoms in children with ASD, with sustained benefits over two years.
- Improvements included better eye contact, communication, and social responsiveness.
Considerations:
- Regulatory status: FMT is approved for treating recurrent C. difficile infections, but not yet standard for ASD.
- Ethical and safety concerns: Screening donors, managing infections, and maintaining procedural quality are critical.
- Long-term effects: Still largely unknown; ongoing trials are essential.
Actionable Insight:
- Follow progress in FMT clinical trials for ASD.
- Consult medical experts before considering FMT in non-trial settings.
- Support regulatory frameworks that allow responsible, evidence-based FMT exploration.
D. Metabolite-Targeted Therapies: Precision Neurochemistry
What’s the Potential?
Given the USC study’s emphasis on kynurenate (KA) and other tryptophan-derived metabolites, future treatments may seek to directly manipulate metabolic pathways that influence neurotransmitter balance.
- Pharmacological agents may be developed to:
- Boost KA synthesis.
- Increase serotonin precursor availability (e.g., 5-HTP).
- Modulate the gut microbiota to influence tryptophan metabolism directionally.
- Nutritional compounds (like vitamin B6, magnesium, and niacin) are already known to play cofactor roles in these pathways and might be optimized for therapeutic benefit.
Future Directions:
- Development of “psychobiotics”—bacteria or compounds designed to improve mental health via gut interactions.
- Integration with genomic and metabolomic profiling to offer highly personalized interventions.
Actionable Insight:
- Advocate for more funding in gut-brain metabolic research.
- Encourage biotech collaborations to translate findings into safe, scalable treatments.
- Monitor research exploring how nutritional psychiatry and microbiome science intersect.
The Holistic Horizon: Integrating Gut Health Into Autism Care
While no single intervention will be a silver bullet, these gut-focused strategies represent an important step forward in multidimensional autism care. They invite us to expand our lens—from treating outward behaviors to addressing internal physiological states that shape those behaviors.
Combining behavioral therapies with nutritional, microbial, and metabolic interventions may unlock new potential for healing, learning, and thriving for individuals on the spectrum.

V. Rethinking Care Models: Integrating Gut Health into Autism Support
To truly support individuals with autism, care models must evolve beyond behavior management to include physiological health—particularly gut health—as a core component of therapy. As emerging research points to the critical role of the gut-brain axis in shaping behavior, emotion, and cognition, we are called to rethink autism care as an interdisciplinary, integrative, and personalized endeavor.
A. The Current Standard of Care: Valuable but Incomplete
Children diagnosed with autism spectrum disorder (ASD) typically receive a multi-pronged intervention strategy that may include:
- Applied Behavior Analysis (ABA): A widely used method focused on reinforcing positive behaviors and reducing problematic ones.
- Speech and Language Therapy: Enhancing communication skills, especially for non-verbal or minimally verbal children.
- Occupational Therapy: Supporting sensory integration, motor skills, and daily living activities.
- Pharmacological Support: Prescribed for co-occurring conditions like anxiety, irritability, ADHD, or seizures.
These interventions are essential, but they often address the “output” of the brain—the behavior—without fully considering the “input”—the physiological state of the body, particularly the gut.
B. The Overlooked Burden: Gastrointestinal Issues in ASD
A growing body of clinical evidence shows that gastrointestinal (GI) symptoms are significantly more prevalent in children with ASD compared to their neurotypical peers.
- Common GI symptoms include:
- Constipation
- Diarrhea
- Abdominal pain
- Gastroesophageal reflux
- Food sensitivities or aversions
Studies suggest that up to 70% of children with autism experience chronic GI distress, yet these issues are often dismissed as secondary or unrelated to core autism symptoms.
Crucially, unresolved GI problems may amplify behavioral difficulties, such as:
- Irritability
- Meltdowns
- Sleep disturbances
- Aggression or withdrawal
Children—especially non-verbal or minimally verbal ones—may express internal discomfort through changes in mood or behavior, often misattributed to “behavioral issues” rather than physical pain.
C. Gut Health as a Clinical Priority in ASD Care
In light of these findings, it is imperative that gut health be treated not as an optional or alternative focus, but as a standard part of autism assessment and care.
Key Recommendations:
- Routine Gut Health Screening:
- Regular checkups should include questions about GI symptoms, bowel patterns, food intake, and appetite changes.
- Use validated tools like the Gastrointestinal Severity Index (GSI) for ASD.
- Functional GI Assessment:
- Stool testing (for microbiome profiling, inflammation, pathogens)
- Bloodwork (to check for nutrient deficiencies, inflammation markers)
- Food sensitivity panels (where appropriate)
- Symptom Management:
- Use evidence-based strategies for relieving constipation, diarrhea, and pain.
- Ensure that diet is tailored to minimize inflammation and discomfort.
- Consider working with GI specialists familiar with autism.
D. Multidisciplinary Collaboration: The Future of ASD Care
No single specialist can manage the complexity of autism in isolation. A truly effective and compassionate care model must include collaboration between:
- Pediatricians: For overall health monitoring and early detection of comorbidities.
- Gastroenterologists: Especially for chronic GI issues and specialized testing.
- Neurologists: To assess brain function, seizures, and developmental pathways.
- Clinical Nutritionists/Dietitians: To design microbiome-supportive, sensory-friendly diets.
- Behavioral and Speech Therapists: To align communication strategies with physiological well-being.
- Occupational Therapists: To address sensory processing issues that may impact feeding and digestion.
This cross-disciplinary model ensures that no signal—verbal, behavioral, or biological—is ignored. It supports the whole child, not just the diagnosis.
E. Individualized Gut-Brain Care Plans: Especially Critical for Non-Verbal Children
Many children with ASD struggle to verbalize pain or discomfort, making them especially vulnerable to undiagnosed or misdiagnosed health issues.
Best Practices:
- Behavior-GI Symptom Logs: Parents and caregivers should track both GI patterns and behavioral shifts to identify correlations.
- Sensory-Informed Diet Planning: Work with OTs and nutritionists to accommodate texture, taste, and smell sensitivities.
- Proactive Screenings: Especially when there is unexplained irritability, sleep issues, or loss of skills.
Case Insight:
One parent’s observation that their child screamed uncontrollably every afternoon led to an eventual diagnosis of abdominal migraines, a gut-brain condition. After implementing a gut-healing diet and probiotic protocol, behavioral symptoms reduced significantly—without any change in behavioral therapy.
A Call to Action: Let the Gut Speak in Autism Care
If we ignore the gut, we risk silencing an entire dimension of the autism experience. By integrating gut health into the standard of care, we open the door to more accurate diagnoses, more effective interventions, and most importantly, better quality of life for autistic individuals and their families.
This is not about replacing existing therapies. It is about expanding our toolbox, embracing biological empathy, and listening to what the body is trying to tell us.
VI. Challenges and the Road Ahead: What We Still Need to Know
While the USC study opens an exciting window into the gut-brain-behavior connection in autism, it is a starting point—not a final answer. To responsibly translate these insights into effective care, we must acknowledge the scientific limitations, ethical complexities, and logistical barriers that currently stand between research findings and real-world applications.
A. Correlation Is Not Causation: The Need for Interventional Proof
The USC study provides compelling evidence of a relationship between altered gut metabolites, changes in brain activity, and ASD behavioral traits. However, the findings are correlational—meaning they show patterns of association, not cause-and-effect.
Why this matters:
- Just because a gut metabolite is lower in children with autism doesn’t mean it causes autism or its symptoms.
- The altered brain activity seen in children with ASD could be the effect, cause, or coincidental by-product of gut changes.
To move from possibility to probability, we need:
- Longitudinal studies: Tracking children over time to observe how gut health shifts correlate with changes in brain function and behavior.
- Controlled intervention trials: Testing whether altering the gut—through diet, probiotics, or other means—directly leads to measurable improvements in ASD symptoms.
B. Sample Size and Demographic Gaps
While scientifically rigorous, the USC study had a limited sample size. Smaller sample groups reduce statistical power and can increase the chance of false positives or overgeneralizations.
Additional research must address:
- Gender diversity: Most autism studies are disproportionately male-focused. Yet, autism can present differently in girls and may have different physiological underpinnings.
- Age stratification: Does the gut-brain relationship change with developmental stages—infancy, adolescence, adulthood?
- Cultural and dietary diversity: Microbiomes are deeply influenced by diet, environment, and lifestyle. Results from one group may not apply universally.
C. The Complexity of the Microbiome: An Ecosystem, Not a Variable
The human gut is home to trillions of microorganisms, interacting with each other and the host body in dynamic and still-mysterious ways. This complexity poses major challenges for research:
- Microbiome variability: No two individuals have the same microbial signature. Even within the same person, the microbiome can fluctuate due to stress, diet, medication, and illness.
- Multifactorial influences: Genes, environment, immune responses, and even prenatal exposures interact with the microbiome in ways we are only beginning to understand.
Advanced tools needed:
- Metagenomics: To identify the specific genes and capabilities of microbial communities—not just which microbes are present.
- Targeted metabolomics: To map the chemical outputs of microbes (e.g., serotonin, KA, short-chain fatty acids) and their effects on the brain.
- Neuroimaging integrations: Future studies should combine microbiome and metabolite data with real-time brain imaging to pinpoint pathways of influence.
D. Ethical and Practical Barriers in Autism Research
Working with autistic individuals—particularly children—raises unique ethical considerations:
- Consent and autonomy: Especially for non-verbal or cognitively impaired individuals, how do we ensure ethical participation?
- Invasiveness: Some procedures (e.g., fecal transplants, repeated blood tests) may be distressing or not well-tolerated.
- Standardization difficulty: Unlike drug trials, microbiome-targeted therapies often require complex, individualized, and diet-dependent protocols that are hard to replicate across studies.
Additionally, the commercialization of microbiome therapies (such as unregulated probiotics or costly “gut healing” programs) risks exploiting vulnerable families searching for hope.
Solution-focused suggestions:
- Encourage open science and non-profit research to make gut-focused care accessible and ethically sound.
- Foster community-partnered research involving autistic individuals and their caregivers in the design and interpretation of studies.
E. From Challenges to Opportunity: Building a Responsible Research Roadmap
The current moment represents a scientific and moral imperative. We must not:
- Overpromise cures,
- Oversimplify mechanisms,
- Or overlook the lived realities of autistic individuals.
But neither should we let complexity paralyze us.
A responsible research agenda includes:
- Funding interdisciplinary studies combining neuroscience, gastroenterology, and behavioral science.
- Diversifying study populations to reflect the true range of autism experience.
- Creating ethical frameworks for involving vulnerable participants with dignity and agency.
- Training professionals to recognize and respond to gut-brain issues in daily autism care.
- Pursuing personalized medicine: Tailoring interventions to an individual’s unique biology, rather than applying one-size-fits-all solutions.
VII. Hope, Holism, and Human-Centered Research
Conclusion First:
The emerging gut-brain research represents more than a scientific breakthrough—it signals a shift toward compassionate, integrative, and human-centered autism care. It reminds us that healing may come not only through labs and therapies, but through understanding the body as a whole system—and each person as more than a diagnosis.
A. Rediscovering the Wisdom of Wholeness
Long before modern medicine, ancient systems like Ayurveda, Traditional Chinese Medicine, and indigenous healing traditions emphasized the unity of body, mind, and environment. Today, neuroscience and microbiology are beginning to validate these holistic insights:
- The gut is not just a digestive organ; it’s a second brain, deeply involved in emotion, immunity, and cognition.
- Neurodevelopmental conditions like autism are not solely psychological or behavioral; they are multi-systemic, involving metabolic, immune, and neural interactions.
This convergence of science and traditional wisdom calls for a new paradigm of autism care—one that doesn’t isolate symptoms but embraces the whole child and the ecosystem they live in.
B. A Turning Point for Families and Clinicians
For countless families and caregivers of individuals with autism, this research offers hope grounded in data—not as a “cure,” but as a path to deeper understanding and more effective support.
- For parents: it validates the gut instincts many have long held—“My child’s tummy issues and meltdowns are connected.”
- For clinicians: it provides a scientific roadmap to address autism’s hidden layers—beyond behavior charts and medication.
- For researchers: it opens a frontier where biology and empathy intersect, inspiring studies that can transform lives, not just lab reports.
C. Toward Integrative and Personalized Autism Care
The future of autism care must be personal, not prescriptive. It must recognize that:
- No two individuals on the spectrum are the same.
- Healing may involve behavioral therapy for one, gut support for another, or ideally—both, in a coordinated plan.
A holistic autism care model would include:
- Behavioral and developmental therapy (ABA, speech, occupational)
- Nutritional guidance and GI support
- Microbiome and metabolic assessments
- Emotional and family-centered care
- Neuroscience-informed interventions
- Collaborative care teams spanning pediatricians, neurologists, dietitians, psychologists, and caregivers.
D. The Way Forward: Curiosity, Compassion, and Collective Action
Advancing care for autism is not just a medical challenge—it is a moral one. The gut-brain story invites us to act with:
- Curiosity: to ask deeper questions, to explore new pathways, to remain open-minded and rigorous.
- Compassion: to center the lived experiences of autistic individuals, respecting their voices, differences, and dignity.
- Collaboration: to bring together parents, professionals, policymakers, and researchers in building systems that heal and empower.
We must fund ethical science, fight misinformation, resist exploitation, and listen deeply—to the gut, the brain, and above all, the human heart.
VIII. Conclusion: Gut, Brain, and the Future of Autism Care
Recap of Learnings:
In this exploration of the groundbreaking USC study and the expanding field of gut-brain research, we’ve uncovered a transformative perspective on autism care—one that invites us to look beyond traditional behavioral models and see the body and brain as deeply interconnected ecosystems. The gut, long underestimated, may hold powerful clues to understanding the inner lives and outer expressions of those with autism spectrum disorder (ASD).
From the tryptophan pathways influencing serotonin production, to the mid-insula and mid-cingulate cortex mediating behavior and sensory experience, science is beginning to paint a holistic, dynamic picture of ASD—one that honors the complexity, individuality, and humanity of each person on the spectrum.
Key Takeaways:
- The gut-brain axis plays a significant role in emotional regulation, cognition, and behavior—domains often affected in autism.
- The USC study establishes a compelling correlation between gut metabolites, brain activity patterns, and ASD symptom severity.
- Future therapies may include dietary interventions, prebiotics/probiotics, fecal microbiota transplantation (FMT), and metabolite-targeted treatments.
- Behavioral therapies remain crucial but could be significantly enhanced by gut-focused, personalized strategies.
- Holistic, multidisciplinary care must become the standard—integrating neurology, nutrition, psychology, and compassionate family support.
Actionables: What You Can Do Next
For Parents and Caregivers:
- Track gastrointestinal symptoms alongside behavioral patterns.
- Consult integrative professionals who understand both neurological and nutritional dimensions.
- Consider exploring anti-inflammatory, fiber-rich diets in consultation with your pediatrician or a clinical nutritionist.
- Ask your care team to include gut health assessments in your child’s support plan.
For Health Professionals:
- Include gut-brain education in autism care protocols.
- Form interdisciplinary teams to address ASD from multiple angles.
- Advocate for research funding in metabolomics, microbiome science, and personalized gut-based interventions.
For Researchers and Students:
- Investigate causal pathways, not just correlations.
- Prioritize longitudinal and diverse population studies.
- Use tools like targeted metabolomics, metagenomics, and serum analyses to deepen the picture.
For Policymakers and Public Health Advocates:
- Support integrative autism care policies that fund nutrition, mental health, and behavioral services as a unit.
- Increase public funding for microbiome research.
- Push for GI screening as part of routine autism evaluations.
Why It Matters: The Role of the Village
Gut health isn’t just a medical issue—it’s a public health, developmental, and social justice issue. The burden of untreated GI symptoms and misunderstood behavior falls not just on the autistic individual, but on families, teachers, and communities who often feel powerless.
But we’re not powerless. By sharing knowledge, fostering collaboration, and promoting whole-person care, we can offer more than symptom management—we can offer dignity, empathy, and a path forward.
Participate and Donate to MEDA Foundation
At the MEDA Foundation, we believe in building self-sustaining ecosystems of care, inclusion, and empowerment for neurodiverse individuals.
Support our mission to:
- Fund community-led gut health awareness programs and nutrition workshops for families and schools.
- Develop assistive technology that aligns with holistic therapies.
- Train local healthcare providers in integrative autism care.
- Create employment pathways for autistic individuals based on their unique strengths.
💡 Your donation fuels transformation.
👐 Your volunteering spreads compassion.
🌱 Your voice creates a movement.
👉 Visit www.MEDA.Foundation to donate, volunteer, or partner with us in creating a world where neurodiversity thrives—body, brain, and beyond.
Book References and Further Reading
- “The Second Brain” by Michael D. Gershon
→ Pioneering look at the enteric nervous system and its influence on behavior. - “Brain Maker” by Dr. David Perlmutter
→ Connects gut microbiome to mental and neurological conditions. - “Gut” by Giulia Enders
→ A delightful, accessible read on why gut health matters more than we think. - “The Mind-Gut Connection” by Dr. Emeran Mayer
→ Explores how microbes communicate with the brain and influence emotions. - Nature Communications Journal
→ For in-depth reading of the original USC study: “Gut metabolites influence brain activity and behavior in children with autism.”